Monthly Archives: April 2020
CNBC’s full interview with Berkshire Hathaway CEO Warren Buffett [Feb 2020]
HM Government Borrowing, March 2020
Another month, guess what, take a lucky guess, it is the same old story, HM Government, spends more money than it receives via taxes and duties.
Another deficit month, thus to bridge the gap, needs to borrow on the bond market In March 2020 , the HM Government had to borrow money to meet the difference between tax revenues and public sector expenditure. The term for this is The PSNCR: The Public Sector Net Cash Requirement. There were “only” 4 auctions of Gilts (UK Government Bonds) by the UK Debt Management Office to raise cash for HM Treasury:-
19-Mar-2020 0 5/8% Treasury Gilt 2025 £3,250.0000 Million
17-Mar-2020 1¾% Treasury Gilt 2049 £2,299.9990 Million
10-Mar-2020 4¾% Treasury Gilt 2030 £2,587.4880 Million
05-Mar-2020 0 1/8% Index-linked Treasury Gilt 2028 3 months £1,244.2380 Million
04-Mar-2020 0 5/8% Treasury Gilt 2025 £3,500.0000 Million
When you add the cash raised:-
£3,250.0000 Million + £2,299.9990 Million+ £2,587.4880 Million + £1,244.2380 Million + £3,500.0000 Million = £12,764.406 Million
£12,881.725 Million = £12.881725 Billion
On another way of looking at it, is in the 31 days in March 2020, HM Government borrowed:- £415.5395161290323 Million each day for the 31 days.
We are fortunate, while the global banking and financial markets still has the confidence in HM Government to buy the Gilts (Lend money to the UK), the budget deficit keeps rising. What is also alarming, is the dates these bond mature 2025, 2028, 2030 and 2049. All long term borrowings, we are mortgaging our futures, but at least “We Are In It Together….”
What coronavirus means for the global economy | Ray Dalio
Newsnight: Emily is superb
“The disease is not a great leveller, the consequences of which everyone – rich or poor – suffers the same,” the 49-year-old said.
“This is a myth which needs debunking. Those on the front line right now – bus drivers and shelf stackers, nurses, care home workers, hospital staff and shop keepers – are disproportionately the lowest paid members of our workforce. They are more likely to catch the disease because they are more exposed.”
And she added: “Those who live in tower blocks and small flats will find the lockdown a lot tougher. Those who work in manual jobs will be unable to work from home.
Covid 19: The University of Liverpool: Funding for Research
Hello.
The University of Liverpool has a world famous School of Tropical Medicine.
https://www.liverpool.ac.uk/coronavirus/support-us/
They are asking for money to help research into Covid 19 (see the link at the bottom)
It was my first University, and I have made a donation to Liverpool’s world leading research.
If you can help, it would be wonderful.
Thanks
Asad
We are all the same
The WorldCON Fraud.
https://www.youtube.com/watch?v=juebYwvU91o
https://www.youtube.com/watch?v=juebYwvU91o
The episode from American Greed on CNBC.
Copper Destroys Viruses and Bacteria. Why Isn’t It Everywhere?
It could destroy norovirus, MRSA, virulent strains of E. coli, and coronaviruses—including the novel strain currently causing the COVID-19 pandemic.
In 1852, physician Victor Burq visited a copper smelter in Paris’s 3rd arrondissement, where they used heat and chemicals to extract the reddish-brown metal. It was a dirty and dangerous job. Burq found the facility to be “in poor condition,” along with the housing and the hygiene of the smelters. Normally, their mortality rates were “pitiful,” he observed.
Yet, the 200 employees who worked there had all been spared from cholera outbreaks that hit the city in 1832, 1849, and 1852. When Burq learned that 400 to 500 copper workers on the same street had also mysteriously dodged cholera, he concluded that something about their professions—and copper—had made them immune to the highly infectious disease. He launched a detailed investigation into other people who worked with copper, in Paris and cities around the world.
In the 1854 to 1855 cholera epidemic, Burq could not find any deaths of jewellers, goldsmiths, or boilermakers—all those who worked with copper. In people in the army, he found that musicians who played brass instruments (brass is partly copper) were also protected.
In the 1865 Paris epidemic, 6,176 people died of cholera, out of a population of 1,677,000 people—that’s 3.7 people out of every 1,000. But of the 30,000 who worked in different copper industries, only 45 died—an average of around 0.5 per 1,000.
After visiting 400 different businesses and factories in Paris, all of which used copper, and collecting reports from England, Sweden, and Russia on more than 200,000 people, he concluded to the French Academies of Science and Medicine in 1867 that “copper or its alloys, brass and bronze, applied literally and pregnantly to the skin in the cholera epidemic are effective means of prevention which should not be neglected.”
Today, we have insight into why a person handling copper day in and day out would have protection from a bacterial threat: Copper is antimicrobial. It kills bacteria and viruses, sometimes within minutes. In the 19th century, exposure to copper would have been an early version of constantly sanitizing one’s hands.
Since then, studies have shown that copper is able to destroy the microbes that most threaten our lives. It has been shown to kill a long list of microbes, including norovirus, MRSA, a staph bacteria that has become resistant to antibiotics, virulent strains of E. coli that cause food-borne illness, and coronaviruses—possibly including the novel strain currently causing the COVID-19 pandemic.
If copper were more frequently used in hospitals, where 1 in 31 people get healthcare-acquired infections (HAI), or in high-traffic areas, where many people touch surfaces teeming with microbial life—it could play an invaluable role in public health, said Michael Schmidt, a professor of microbiology and immunology at the Medical University of South Carolina, who studies copper. And yet, it is woefully absent from our public spaces, healthcare settings, and homes.
“What happened is our own arrogance and our love of plastic and other materials took over,” Schmidt said of the cheaper products more frequently used. “We moved away from copper beds, copper railings, and copper door knobs to stainless steel, plastic, and aluminum.”
Many of the microbes that make us sick can live on hard surfaces for up to four or five days. When we touch those surfaces, the microbes can make it into our bodies through our nose, mouth, or eyes, and infect us.
On copper surfaces, bacteria and viruses die. When a microbe lands on a copper surface, the copper releases ions, which are electrically charged particles. Those copper ions blast through the outer membranes and destroy the whole cell, including the DNA or RNA inside. Because their DNA and RNA are destroyed, it also means a bacteria or virus can’t mutate and become resistant to the copper, or pass on genes (like for antibiotic resistance) to other microbes.
Before people even knew what bacteria and viruses were, they knew that copper could—somehow—ward off infection. The first recorded medical use of copper is from one of the oldest-known books, the Smith Papyrus, written between 2600 and 2200 B.C. It said that copper was used to sterilize chest wounds and drinking water. Egyptian and Babylonian soldiers would similarly put the shavings from their bronze swords (made from copper and tin) into their open wounds to reduce infections. A more contemporary use of copper: In New York City’s Grand Central Station, the grand staircase is flanked by copper handrails. “Those are actually anti-microbial,” Schmidt said.
The copper smelters were, ostensibly, exposed to less of the cholera bacterium because their surroundings included a lot of copper that bacteria couldn’t live on. That and they potentially were covered in copper particles. If metallurgy doesn’t call to you, there are now some products that are advertised as “copper hand sanitizers,” but they work only if you can expose every surface of your hands to the copper for at least a full minute—essentially transferring any microbes to the copper surface to be killed. It could be difficult to get to every part of your skin’s surface, so having copper surfaces in your environment paired with handwashing would be the ideal combination.
Schmidt said that using copper along with standard hygiene protocols has been shown to reduce bacteria in health care settings by 90 percent. A study from 1983 found that hospital door knobs made of brass, which is part copper, barely had any E. coli growth on them, compared to stainless steel knobs which were “heavily colonized.” This is significant because of how rampant healthcare-acquired infections are: In the U.S. alone, there are about 1.7 million infections and 99,000 deaths linked to HAIs per year, which cost between $35.7 and $45 billion annually, from the extra treatments people need when they get infected.
Microbes that live on surfaces in patient rooms and common spaces in hospitals play a role in getting a HAI—and this is where copper could help. And during this pandemic, when there is serious concern about the spread of the novel coronavirus via contaminated surfaces, a virus-killing substance seems worthwhile indeed.
A study from 2015 found that a different coronavirus, human coronavirus 229E, which causes respiratory tract infections, could still infect a human lung cell after five days of being on materials like teflon, ceramic, glass, silicone rubber, and stainless steel. But on copper alloys, the coronavirus was “rapidly inactivated.”
In a new preprint on SARS-CoV2, the strain that causes COVID-19, researchers at the National Institutes of Health virology laboratory in Montana sprayed the virus onto seven different common materials, reported MIT Technology Review. They found that it survived the longest—up to three days—on plastic and stainless steel, suggesting that surfaces in hospitals or steel poles on public transit could be places where people pick up the illness. Just a single droplet from a cough or sneeze can carry an infectious dose of a virus.
Bill Keevil, a professor of environmental healthcare at the University of Southampton in England who has previously received funding from the Copper Development Association, said that if copper surfaces were put in communal areas where many people gather, it could help reduce the transmission of respiratory viruses, like coronavirus 229E and also SARS-CoV2. Other than hospitals, he thinks the ideal locations for copper are public transportation systems, like buses, airports, subways. But he doesn’t stop there: He would also like to see copper used in sports equipment in gyms, like weights, along with other everyday objects, including shared office supplies, like pens.
In the preprint, SARS-CoV2 “liked copper least,” Antonio Regalado wrote in MIT Technology Review. “The virus was gone after just four hours.”
In 2012, Schmidt and his colleagues ran a clinical trial in three hospitals, Memorial Sloan Kettering Cancer Center in New York City, Medical University of South Carolina, in Charleston, and Ralph H. Johnson Veterans Administration Medical Center, also in Charleston.
First, they figured out which items closest to a patient were the most contaminated with microbes—those were the bed rails, the nurse call button, the arm of the visitor chair, the tray tables, and the IV pole. Enveloping these items in copper reduced the presence of microbes by 83 percent. As a result, HAIs were reduced by 58 percent, even though the researchers had introduced copper to less than 10 percent of the surface area of the room.
We have other methods of killing bacteria and viruses to mitigate HAIs, including ultraviolet light and hydrogen peroxide gas. But both require a hospital room to be empty, and once sick people re-enter rooms, surfaces can easily be contaminated again. “Copper is continuously working 24/7 without supervision, without any need to intervene, and it never runs out,” Schmidt said. “As long as the metal’s there, it’s good to go.”
So given how well it could work, for hospital infections and for health more generally, why isn’t copper everywhere? Why isn’t every door knob, every subway rail, every ICU room, made of copper? Why can we easily buy stainless steel water bottles, but not copper? Where are the copper iPhone cases?
It doesn’t seem like we’ll run out of copper in the near future, according to the World Copper Factbook from 2019. Copper is one of the most recycled of all metals—nearly all copper can be recycled and not lose any of its properties.
Doctors and healthcare workers might not be aware of its properties, as Keevil wrote in The Conversation: “When doctors are asked to name an antimicrobial metal used in healthcare, the most common reply is silver—but little do they know that silver does not work as an antimicrobial surface when dry—moisture needs to be present.”
There might also be a perception that copper is too expensive, Schmidt said, despite the fact that the numbers indicate it would ultimately save money. One of Keevil and Schmidt’s studies from 2015 did the math: The cost of treating an HAI ranges from $28,400 to $33,800 per patient. Installing copper on 10 percent of surfaces cost $52,000 and prevented 14 infections over the course of the 338-day study. If you take the lower end of the HAI treatment cost ($28,400), then those 14 prevented infections saved a total of $397,600, or $1,176 a day.
Even when factoring in how much the copper cost initially, you’d make that money back in savings within two months, Schmidt said. And considering that the copper never loses its microbial killing abilities—hospitals would quickly be saving money (and lives).
“Your payback is literally in less than two [prevented] infections,” he said. “I really struggle with this. Since 2013, I have been literally begging, groveling, pleading, with any and all concerned to make a completely copper encapsulated
[hospital]
bed.”
Advertisement
He recently did convince a company to invest, and said they’re in the process of testing it to show that it could reduce infections even further than 58 percent.
Another reason copper may have been passed over for steel, plastic, or glass is that it can easily tarnish and requires a lot of cleaning to remain shiny. “But copper is antimicrobial regardless of how grody it looks, if it turns green on you, it still has the ability to kill bacteria and viruses and fungi,” he said.
Some places around the world have started to use copper. In Chile, a theme park called Fantasilandia, replaced a lot of its commonly touched surfaces with copper. At the Atlanta airport, 50 water bottle filling stations are now made with copper. But Schmidt believes it should be more widespread.
He said that one of the reasons scientists are worried about the current coronavirus is how infectious it is, and a major way people might be getting it is from touching contaminated surfaces. He thinks it’s possible that the pandemic could raise awareness for copper—if it motivates anyone to start using it. Imagine, he said, if our hospitals and public spaces already had copper in place—it’s impossible to say for sure, but it’s likely that transmission would have been affected.
“I have great confidence that it would work because bacteria or viruses are the ones causing the infection,” hesaid. “If their numbers go down, common sense would tell you: you should have fewer infections.”
Investing in Light

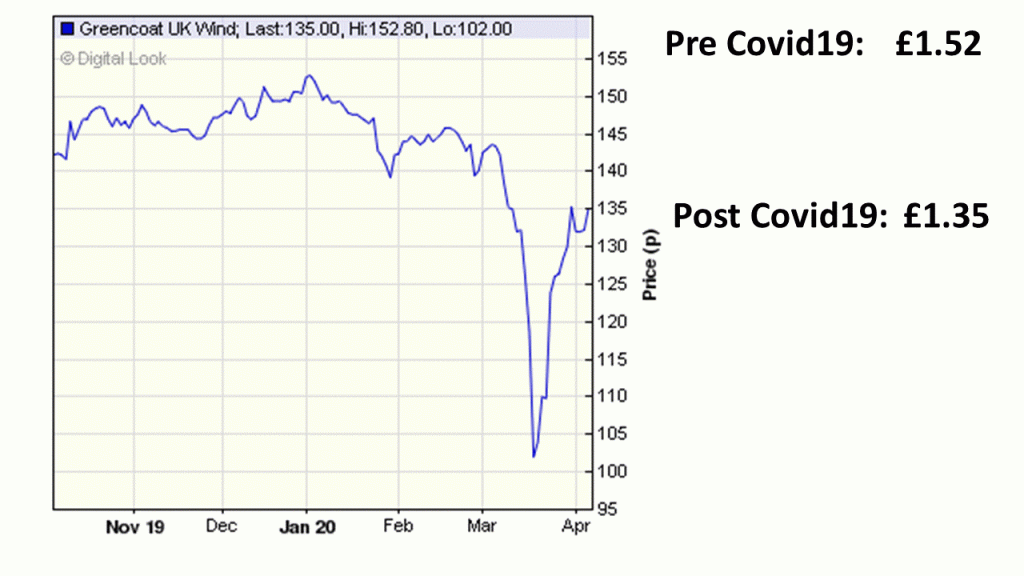
Greencoat UK Wind: Price Drop from Covid 19
The current pandemic crisis over Covid 19 is having major effects on the financial markets.
Take the UK Renewable s company, UK Greencoat Wind.
http://www.shareshop.hsbc.co.uk/shareshop/security.cgi?csi=7013076&action=
It has seen its share price drop from £1.52 to £1.35, but a quick recovery in a sector that is not violently affected by the Covid19 lockdown.
The Two Brilliant Investors

Fighting Covid19
Tip of the iceberg’: is our destruction of nature responsible for Covid-19?
As habitat and biodiversity loss increase globally, the coronavirus outbreak may be just the beginning of mass pandemics
Mayibout 2 is not a healthy place. The 150 or so people who live in the village, which sits on the south bank of the Ivindo River, deep in the great Minkebe Forest in northern Gabon, are used to occasional bouts of diseases such as malaria, dengue, yellow fever and sleeping sickness. Mostly they shrug them off.
But in January 1996, Ebola, a deadly virus then barely known to humans, unexpectedly spilled out of the forest in a wave of small epidemics. The disease killed 21 of 37 villagers who were reported to have been infected, including a number who had carried, skinned, chopped or eaten a chimpanzee from the nearby forest.
I travelled to Mayibout 2 in 2004 to investigate why deadly diseases new to humans were emerging from biodiversity “hotspots” such as tropical rainforests and bushmeat markets in African and Asian cities.
It took a day by canoe and then many hours along degraded forest logging roads, passing Baka villages and a small goldmine, to reach the village. There, I found traumatised people still fearful that the deadly virus, which kills up to 90% of the people it infects, would return.
Villagers told me how children had gone into the forest with dogs that had killed the chimp. They said that everyone who cooked or ate it got a terrible fever within a few hours. Some died immediately, while others were taken down the river to hospital. A few, like Nesto Bematsick, recovered. “We used to love the forest, now we fear it,” he told me. Many of Bematsick’s family members died.
Only a decade or two ago it was widely thought that tropical forests and intact natural environments teeming with exotic wildlife threatened humans by harbouring the viruses and pathogens that lead to new diseases in humans such as Ebola, HIV and dengue.
But a number of researchers today think that it is actually humanity’s destruction of biodiversity that creates the conditions for new viruses and diseases such as Covid-19, the viral disease that emerged in China in December 2019, to arise – with profound health and economic impacts in rich and poor countries alike. In fact, a new discipline, planetary health, is emerging that focuses on the increasingly visible connections between the wellbeing of humans, other living things and entire ecosystems.
Is it possible, then, that it was human activity, such as road building, mining, hunting and logging, that triggered the Ebola epidemics in Mayibout 2 and elsewhere in the 1990s and that is unleashing new terrors today?
“We invade tropical forests and other wild landscapes, which harbour so many species of animals and plants – and within those creatures, so many unknown viruses,” David Quammen, author of Spillover: Animal Infections and the Next Pandemic, recently wrote in the New York Times. “We cut the trees; we kill the animals or cage them and send them to markets. We disrupt ecosystems, and we shake viruses loose from their natural hosts. When that happens, they need a new host. Often, we are it.”
Increasing threat
Research suggests that outbreaks of animal-borne and other infectious diseases such as Ebola, Sars, bird flu and now Covid-19, caused by a novel coronavirus, are on the rise. Pathogens are crossing from animals to humans, and many are able to spread quickly to new places. The US Centers for Disease Control and Prevention (CDC) estimates that three-quarters of new or emerging diseases that infect humans originate in animals.
Some, like rabies and plague, crossed from animals centuries ago. Others, such as Marburg, which is thought to be transmitted by bats, are still rare. A few, like Covid-19, which emerged last year in Wuhan, China, and Mers, which is linked to camels in the Middle East, are new to humans and spreading globally.
Other diseases that have crossed into humans include Lassa fever, which was first identified in 1969 in Nigeria; Nipah from Malaysia; and Sars from China, which killed more than 700 people and travelled to 30 countries in 2002–03. Some, like Zika and West Nile virus, which emerged in Africa, have mutated and become established on other continents.
Kate Jones, chair of ecology and biodiversity at UCL, calls emerging animal-borne infectious diseases an “increasing and very significant threat to global health, security and economies”.
Amplification effect
In 2008, Jones and a team of researchers identified 335 diseases that emerged between 1960 and 2004, at least 60% of which came from animals.
Increasingly, says Jones, these zoonotic diseases are linked to environmental change and human behaviour. The disruption of pristine forests driven by logging, mining, road building through remote places, rapid urbanisation and population growth is bringing people into closer contact with animal species they may never have been near before, she says.
The resulting transmission of disease from wildlife to humans, she says, is now “a hidden cost of human economic development. There are just so many more of us, in every environment. We are going into largely undisturbed places and being exposed more and more. We are creating habitats where viruses are transmitted more easily, and then we are surprised that we have new ones.”
ones studies how changes in land use contribute to the risk. “We are researching how species in degraded habitats are likely to carry more viruses which can infect humans,” she says. “Simpler systems get an amplification effect. Destroy landscapes, and the species you are left with are the ones humans get the diseases from.”
“There are countless pathogens out there continuing to evolve which at some point could pose a threat to humans,” says Eric Fevre, chair of veterinary infectious diseases at the University of Liverpool’s Institute of Infection and Global Health. “The risk [of pathogens jumping from animals to humans] has always been there.”
The difference between now and a few decades ago, Fevre says, is that diseases are likely to spring up in both urban and natural environments. “We have created densely packed populations where alongside us are bats and rodents and birds, pets and other living things. That creates intense interaction and opportunities for things to move from species to species,” he says.
Tip of the iceberg
“Pathogens do not respect species boundaries,” says disease ecologist Thomas Gillespie, an associate professor in Emory University’s department of environmental sciences, who studies how shrinking natural habitats and changing behaviour add to the risk of diseases spilling over from animals to humans.
“I am not at all surprised about the coronavirus outbreak,” he says. “The majority of pathogens are still to be discovered. We are at the very tip of the iceberg.”
Humans, says Gillespie, are creating the conditions for the spread of diseases by reducing the natural barriers between host animals – in which the virus is naturally circulating – and themselves. “We fully expect the arrival of pandemic influenza; we can expect large-scale human mortalities; we can expect other pathogens with other impacts. A disease like Ebola is not easily spread. But something with a mortality rate of Ebola spread by something like measles would be catastrophic,” Gillespie says.
Wildlife everywhere is being put under more stress, he says. “Major landscape changes are causing animals to lose habitats, which means species become crowded together and also come into greater contact with humans. Species that survive change are now moving and mixing with different animals and with humans.”
Gillespie sees this in the US, where suburbs fragment forests and raise the risk of humans contracting Lyme disease. “Altering the ecosystem affects the complex cycle of the Lyme pathogen. People living close by are more likely to get bitten by a tick carrying Lyme bacteria,” he says.
Yet human health research seldom considers the surrounding natural ecosystems, says Richard Ostfeld, distinguished senior scientist at the Cary Institute of Ecosystem Studies in Millbrook, New York. He and others are developing the emerging discipline of planetary health, which looks at the links between human and ecosystem health.
“There’s misapprehension among scientists and the public that natural ecosystems are the source of threats to ourselves. It’s a mistake. Nature poses threats, it is true, but it’s human activities that do the real damage. The health risks in a natural environment can be made much worse when we interfere with it,” he says.
Ostfeld points to rats and bats, which are strongly linked with the direct and indirect spread of zoonotic diseases. “Rodents and some bats thrive when we disrupt natural habitats. They are the most likely to promote transmissions [of pathogens]. The more we disturb the forests and habitats the more danger we are in,” he says.
Felicia Keesing, professor of biology at Bard College, New York, studies how environmental changes influence the probability that humans will be exposed to infectious diseases. “When we erode biodiversity, we see a proliferation of the species most likely to transmit new diseases to us, but there’s also good evidence that those same species are the best hosts for existing diseases,” she wrote in an email to Ensia, the nonprofit media outlet that reports on our changing planet.
The market connection
Disease ecologists argue that viruses and other pathogens are also likely to move from animals to humans in the many informal markets that have sprung up to provide fresh meat to fast-growing urban populations around the world. Here, animals are slaughtered, cut up and sold on the spot.
The “wet market” (one that sells fresh produce and meat) in Wuhan, thought by the Chinese government to be the starting point of the current Covid-19 pandemic, was known to sell numerous wild animals, including live wolf pups, salamanders, crocodiles, scorpions, rats, squirrels, foxes, civets and turtles.
Equally, urban markets in west and central Africa sell monkeys, bats, rats, and dozens of species of bird, mammal, insect and rodent slaughtered and sold close to open refuse dumps and with no drainage.
“Wet markets make a perfect storm for cross-species transmission of pathogens,” says Gillespie. “Whenever you have novel interactions with a range of species in one place, whether that is in a natural environment like a forest or a wet market, you can have a spillover event.”
The Wuhan market, along with others that sell live animals, has been shut by the Chinese authorities, and last month Beijing outlawed the trading and eating of wild animals except for fish and seafood. But bans on live animals being sold in urban areas or informal markets are not the answer, say some scientists.
“The wet market in Lagos is notorious. It’s like a nuclear bomb waiting to happen. But it’s not fair to demonise places which do not have fridges. These traditional markets provide much of the food for Africa and Asia,” says Jones.
“These markets are essential sources of food for hundreds of millions of poor people, and getting rid of them is impossible,” says Delia Grace, a senior epidemiologist and veterinarian with the International Livestock Research Institute, which is based in Nairobi, Kenya. She argues that bans force traders underground, where they may pay less attention to hygiene.
evre and colleague Cecilia Tacoli, principal researcher in the human settlements research group at the International Institute of Environment and Development (IIED), argue in a blog post that rather than pointing the finger at wet markets, we should look at the burgeoning trade in wild animals.
“It is wild animals rather than farmed animals that are the natural hosts of many viruses,” they write. “Wet markets are considered part of the informal food trade that is often blamed for contributing to spreading disease. But … evidence shows the link between informal markets and disease is not always so clear cut.”
Changing behaviour
So what, if anything, can we do about all of this?
Jones says that change must come from both rich and poor societies. Demand for wood, minerals and resources from the global north leads to the degraded landscapes and ecological disruption that drives disease, she says. “We must think about global biosecurity, find the weak points and bolster the provision of health care in developing countries. Otherwise we can expect more of the same,” she adds.
“The risks are greater now. They were always present and have been there for generations. It is our interactions with that risk which must be changed,” says Brian Bird, a research virologist at the University of California, Davis School of Veterinary Medicine One Health Institute, where he leads Ebola-related surveillance activities in Sierra Leone and elsewhere.
“We are in an era now of chronic emergency,” Bird says. “Diseases are more likely to travel further and faster than before, which means we must be faster in our responses. It needs investments, change in human behaviour, and it means we must listen to people at community levels.”
Getting the message about pathogens and disease to hunters, loggers, market traders and consumers is key, Bird says. “These spillovers start with one or two people. The solutions start with education and awareness. We must make people aware things are different now. I have learned from working in Sierra Leone with Ebola-affected people that local communities have the hunger and desire to have information,” he says. “They want to know what to do. They want to learn.”
Fevre and Tacoli advocate rethinking urban infrastructure, particularly within low-income and informal settlements. “Short-term efforts are focused on containing the spread of infection,” they write. “The longer term – given that new infectious diseases will likely continue to spread rapidly into and within cities – calls for an overhaul of current approaches to urban planning and development.”
The bottom line, Bird says, is to be prepared. “We can’t predict where the next pandemic will come from, so we need mitigation plans to take into account the worst possible scenarios,” he says. “The only certain thing is that the next one will certainly come.”
This piece is jointly published with Ensia
BP Quarterly Dividend
On Friday 27th March (last week), BP paid out its quarterly dividend.
8.1558p a share.
https://www.londonstockexchange.com/exchange/news/market-news/market-news-detail/BP./14442748.html
The total number of voting rights in BP p.l.c. is 20,261,183,605
Thus:-
20,261,183,605 x £0.081558 = £1,652,461,612.45659
That is £1,652 Million = £1.652 Billion
6.38% dividend yield.